Medical Electrical Equipment Testing & Certification
Medical Electrical Equipment Testing & Certification
- TESTING & CERTIFICATION
- PRODUCT AREAS
- ACCREDITATIONS
- ISO/IEC 17020 Inspection Body (SCC) – Burnaby
- ISO/IEC 17025 Electrical Testing (IAS) – Burnaby
- ISO/IEC 17025 Electrical Testing (IAS) – California
- ISO/IEC 17025 Electrical Testing (IAS) – California
- Nationally Recognized Testing Laboratory (OSHA) – NRTL – Burnaby
- ISO/IEC 17065 Certification Body (IAS) – Burnaby
- ISO/IEC 17065 Certification Body (SCC) – Burnaby
- CB Testing Laboratory (CBTL) – California
- CB Testing Laboratory (CBTL) – Burnaby
- CONTACT US
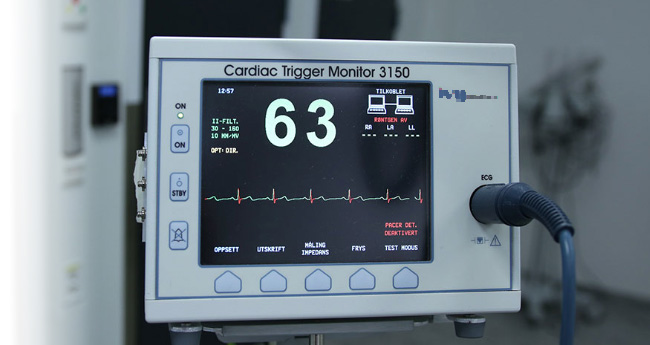
Compliance Experts in Testing and Certification for Medical Electrical Devices
With over 25 years in the market, QAI is your one-stop-shop for Medical Electrical Equipment Testing and Certification. Our expert team ensures your medical devices comply with the highest electrical safety standards. We offer tailored solutions to meet your specific needs, backed by responsive communication throughout the testing process. Trust QAI to provide the precision and expertise required for the electrical safety certification of your medical devices, ensuring they are safe and compliant for use.
What is Medical Electrical Equipment Testing & Certification?
Medical Electrical Equipment Testing & Certification involves a comprehensive evaluation of medical devices to ensure they meet stringent electrical safety standards. This process is crucial for checking that health equipment operates safely and effectively, minimizing risks to patients and healthcare providers. Our testing includes rigorous checks using advanced electrical safety analyzers, ensuring compliance with industry regulations and standards. By achieving certification, medical equipment are validated for safe usage in various healthcare settings, enhancing their reliability and performance. At QAI, we provide expert testing and certification services to guarantee your medical devices meet all necessary safety requirements.
Relevant Standards for Medical Electrical Equipment Testing
These are the critical standards for medical electrical equipment.
EN 60601 - Medical Electrical Equipment
EN 60601 is the primary standard for medical electrical equipment. It sets out the general requirements for basic safety and essential performance of medical devices. This comprehensive standard ensures that medical equipment is designed and tested to protect patients and operators from electrical, mechanical, and thermal hazards. Compliance with EN 60601 is crucial for the approval and market entry of medical devices.
ISO 14971 - Risk Management
ISO 14971 provides a framework for the risk management of medical devices. It outlines the process of identifying, evaluating, and controlling risks associated with medical equipment throughout its life cycle. This standard ensures that potential hazards are systematically assessed and mitigated, contributing to the safety and efficacy of the device.
IEC 60601 or IEC 60601-1
IEC 60601, specifically IEC 60601-1, defines the general requirements for the safety and essential performance of medical electrical equipment. This international standard covers a wide range of safety aspects, including electrical, mechanical, and environmental considerations, to ensure that medical devices operate safely under normal and fault conditions.
IEC 60601-1-2 EMC
IEC 60601-1-2 focuses on the electromagnetic compatibility (EMC) of medical electrical equipment. This standard specifies the requirements and tests for ensuring that medical devices do not emit electromagnetic interference that could affect other equipment and are immune to interference from other devices. Compliance with IEC 60601-1-2 is essential for maintaining the reliable operation of medical devices in various environments.
IEC 60601-1-6 Usability
IEC 60601-1-6 addresses the usability of medical electrical equipment. It sets requirements for the design and evaluation of devices to ensure they are user-friendly and can be operated safely and effectively by intended users in clinical settings. This standard aims to reduce the risk of user errors that could compromise patient safety.
IEC 62366 - Usability Process for Medical Devices
IEC 62366 provides guidelines for the application of usability engineering to medical devices. It outlines the process for identifying user needs, designing user interfaces, and validating that the device can be used safely and effectively. This standard emphasizes the importance of considering the user’s perspective in the design and development of medical equipment.
IEC 62304 - Software Life Cycle for Medical Equipment
IEC 62304 defines the requirements for the life cycle processes of medical device software. It covers the development, maintenance, and risk management of software used in medical devices, ensuring that software is designed and maintained to meet high safety and quality standards. Compliance with IEC 62304 is critical for devices that rely on software to perform essential functions.
Benefits of Using QAI for Your Medical Electrical Equipment Testing & Certification
Choosing QAI for your Medical Electrical Equipment Testing and Certification comes with numerous benefits:
- Expert Knowledge: Our team of highly trained professionals has over 25 years of experience in the field, ensuring your devices are tested by experts.
- Tailored Solutions: We provide customized testing solutions that cater to the specific needs of your medical devices, ensuring precise and accurate results.
- Responsive Communication: Our dedicated customer service team offers prompt and clear communication throughout the testing process, keeping you informed at every step.
- Compliance Assurance: We ensure that your medical devices meet all relevant electrical safety standards and regulations, providing peace of mind and regulatory compliance.
- State-of-the-Art Equipment: Utilizing advanced electrical safety analyzers and testing equipment, we deliver reliable and thorough testing services.
- Safety Certification: Our certification process confirms that your medical devices are safe for use, enhancing their credibility and marketability.
- Comprehensive Testing Services: From electrical safety checks to full certification, we offer a wide range of services to meet all your testing needs.
By partnering with QAI, you ensure that your medical devices not only comply with regulatory requirements but also achieve the highest levels of safety and performance.
Typical Products We Test
Ultrasonic Diagnostic Units

Portable Medical Equipment
Hospital Patient Monitors

Medical Infusion Pumps
Wide Scope of Accreditation at your Services
Learn More About QAI Electrical Safety Services
Beyond Medical Electrical Equipment Safety
In addition to Electrical Safety Testing, Inspection and Certification, QAI also offers Electromagnetic Compatibility Testing, electrical special inspections, electrical field evaluation and hazardous location electrical safety inspections.
Utilizing QAI’s expertise, efficient turnaround times, and dedicated customer service, ensures your medical devices meet regulatory standards promptly and effectively. This facilitates a quicker market entry while maintaining adherence to the highest safety and compliance requirements.
For more information about our services please contact us at:
📞 USA 888.540.4024 📞 CANADA 877.461.8378

Founded in 1995 by a group of experienced certification and testing experts, QAI is an independent third-party testing, inspection and certification organization which serves the building industry, government and individuals with cost effective solutions through our global, in-house capabilities / services.
Latest News
-
Join QAI’s Impartiality Committee!
Be the Voice of Impartiality – Join QAI’s Impartiality Committee! Are you
19 December, 2024 -
2023 Alberta Edition of the National Building Code
Dear Valued Client, Subject: Announcing the publication of the 2023 Alberta Edition
5 April, 2024 -
QAI LABORATORIES (QAI) AND ATRONA TEST LABS (ATL) JOIN FORCES TO EXPAND TESTING CAPABILITY
QAI Laboratories is pleased to announce the acquisition of ATRONA Test Labs,
18 March, 2024
Services
Contact
 USA 1(888)540.4024
USA 1(888)540.4024
 Canada 1(877)461.8378
Canada 1(877)461.8378
 Europe info@qai.org
Europe info@qai.org
 China china-info@qai.org
China china-info@qai.org
 S Korea asia-info@qai.org
S Korea asia-info@qai.org





