Health Care & Medical Device Testing Services
EMC Testing for Health Care & Medical Devices. Electromagnetic Compatibility and Electrical Safety Laboratory.
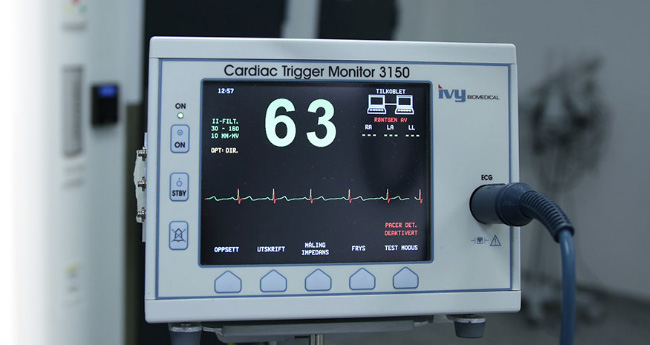
New medical electronic/electrical equipment contributes to more effective healthcare and a better quality of life for all of us. Healthcare technology innovation has a positive impact on our future generations, but it also poses significant challenges to manufacturers and designers in terms of EMC and electrical safety compliance.
The primary compliance requirements for medical devices across various markets include electromagnetic compatibility (EMC) and electrical safety. The fundamental standard in use, with its latest revision, is IEC 60601. Initially published in 1977 as the general standard for medical devices’ safety and essential performance, IEC 60601 has undergone numerous revisions and is widely acknowledged as a benchmark standard.. IEC 60601 consists of over 10 collateral and over 80 standards, it has been adapted by many countries around the world. For the European Union it was EN 60601, for the USA UL2601-1, for Canada it was CSA C22.2 and for Australia and New Zealand it was AS/NZ 3200-1.
The third edition of IEC 60601-1 was published in 2005. The United Sates adapted it in the same year and European Union adapted in 2006. The most important addition to the third edition was risk management and the concept of essential performance. FDA recognized 60601-1-2 Edition 4 was adapted in 2018 for the US market. The Edition 4 covers all the needed EMC requirements for the US market. Medical devices were required to comply with the 4th edition of IEC 60601-1-2 by December 31, 2018, for Europe, the United States, and Canada.
The European Union’s Medical Device Regulation known as EU MDR directive 2017/745 was published in the Official Journal in April of 2017. It supersedes the Medical Devices Directive 93/42/EEC and Active Implantable Medical Devices Directive 90/385/EEC.
Wireless Medical Devices
In addition to this, wireless devices are becoming more prevalent in the medical industry. Therefore, the FDA’s guidance of Radio Frequency Wireless Technology in medical devices must be followed. It can be over whelming with many medical technologies, directives, and hundreds of standards for any manufacturer. This is where you can count on QAI’s team of experts to help you navigate the best path for your compliance needs. On top of the technical standards, manufacturers must follow quality management standards like ISO 13485 and FDA 21 CFR 820.30. These two standards refer to the management system guidelines and processes to be followed by the manufacturer.
QAI is a Telecommunication Certification Body for the US and Conformity Body for Canada. Under one roof, at QAI’s state of the art facilities, you can accomplish the testing and certification of wireless radios for the medical devices, the testing of medical devices by themselves, and FDA’s wireless coexistence testing and electrical safety testing.
QAI’s knowledgeable engineers will help you navigate the path to compliance in an efficient, friendly and professional manner so you can get your product to market as efficiently as possible.
Our capabilities for MEE
Medical Electrical Equipment within QAI’s capability includes:
- Home Healthcare Equipment
- Control Equipment
- Endoscopic Equipment
- Hospital Beds and Chairs
- Laboratory and Measurement Equipment
- Medical Warming / Cooling Cabinets
- Patient Monitors
- Pumps
- Surgical, Cosmetic, and Therapeutic Laser Equipment
- Surgical Luminaires
- Ultrasound and Imaging Equipment
- Wireless Device Technology

Keratometer
Electric Hospital Beds
Medical Lighting
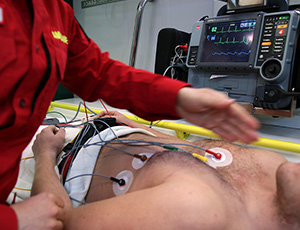
High Acuity Monitors
Why Choose QAI for Medical Electrical Device Compliance?
To certify medical devices to the IEC 60601 standard involves ensuring compliance with specific electromagnetic compatibility (EMC) and safety requirements. We will continue into the description of certifying medical devices for the electrical safety as well. It will have all the details in the electrical safety department.
Electromagnetic Compatibility (EMC) Requirements:
- EMC Risk Management (Manufacturer/OEM):
- Perform a risk analysis to identify potential electromagnetic interference (EMI) risks and establish risk mitigation strategies. ISO 14971 is an international standard for risk management of medical devices and is recognized by the US Food and Drug Administration (FDA), European authorities, Health Canada, the Australia Therapeutic Goods Administration, including other regulatory bodies as the “de facto” standard for risk management.
- EMC Essential Performance (Manufacturer/OEM):
- This is also part of the risk assessment as per ISO 14971. The manufacturers to establish performance limits, and to look at essential performance characteristics in the case of abnormal or faulty conditions. In addition to these changes, essential performance is now a test criterion in assessing if a hazard is present after a specific test.
- EMC Testing (QAI):
- Conduct EMC testing according to IEC 60601 requirements, including radiated and conducted emissions and immunity testing. If necessary, conduct AIM 735731, susceptibility relating to RFIDs and wireless coexistence tests.
- Evaluate the device’s susceptibility to external electromagnetic fields.
- Immunity Testing (QAI):
- Assess the device’s immunity to electromagnetic disturbances, such as electrostatic discharge (ESD), radio-frequency electromagnetic fields (RF), electrical fast transients (EFT), and surge. If necessary, conduct AIM 735731, susceptibility relating to RFIDs and wireless coexistence tests.
- Test conducted and radiated immunity.
- EMI Labeling (Manufacturer/OEM):
- Ensure proper labeling on the device regarding EMC compliance.
For Safety Requirements please visit QAI’s Electrical Safety Portal.
QAI is recognized and holds accreditations from various organizations specific testing, inspection, and certification activities, including: Occupational Safety and Health Administration (OSHA) in the USA as a Nationally Recognized Testing Laboratory (NRTL) and the Standards Council of Canada (SCC) as a certification body. We are also a Certification Body Testing Laboratory (CBTL) under IECEE CB Scheme. QAI’s EMC Lab is accredited by A2LA to ISO/IEC 17025 and registered with FCC, Innovation, Science and Economic Development (ISED) Canada and many other international government bodies. Please visit our accreditations page for details of how we can support in bringing your product to the global market.
QAI follows strict international standards to ensure testing is performed accurately and by knowledgeable staff. QAI will work with you to develop a test plan upfront covering the performance, compatibility, EMC and electrical safety compliance requirements so you know exactly what to expect and the associated costs. We believe in transparency, honesty and being responsive to our clients.
For more information about our services please contact us at:
📞 USA 888.540.4024 📞 CANADA 877.461.8378

Founded in 1995 by a group of experienced certification and testing experts, QAI is an independent third-party testing, inspection and certification organization which serves the building industry, government and individuals with cost effective solutions through our global, in-house capabilities / services.
Latest News
-
Join QAI’s Impartiality Committee!
Be the Voice of Impartiality – Join QAI’s Impartiality Committee! Are you
19 December, 2024 -
2023 Alberta Edition of the National Building Code
Dear Valued Client, Subject: Announcing the publication of the 2023 Alberta Edition
5 April, 2024 -
QAI LABORATORIES (QAI) AND ATRONA TEST LABS (ATL) JOIN FORCES TO EXPAND TESTING CAPABILITY
QAI Laboratories is pleased to announce the acquisition of ATRONA Test Labs,
18 March, 2024
Services
Contact
 USA 1(888)540.4024
USA 1(888)540.4024
 Canada 1(877)461.8378
Canada 1(877)461.8378
 Europe info@qai.org
Europe info@qai.org
 China china-info@qai.org
China china-info@qai.org
 S Korea asia-info@qai.org
S Korea asia-info@qai.org



